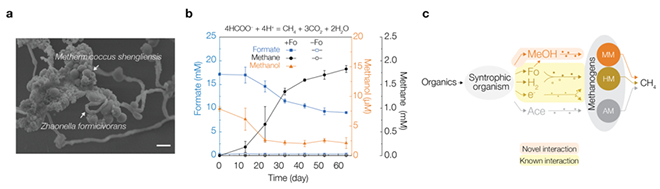
Fig. Syntrophic degradation of formate into methane via interspecies methanol transfer between methanol-generating bacteria and methyl-utilizing methanogenic archaea.
(a) Microscopic image of the co-culture of methanol-generating bacteria Zhaonella and methylotrophic methanogen Methermicoccus. Scale bar = 1 µm.
(b) When Zhaonella and Methermicoccus are incubated with formate, the bacterium converts formate to methanol, which is then transferred to the archaeon to be metabolized for methane (CH4) production. Fo, formate.
(c) Schematic of methanol’s role in syntrophic methanogenic organics degradation. Fo, formate; Ac, acetate; AM, acetoclastic methanogens; HM, hydrogenotrophic methanogens; MM, methylotrophic methanogens.
Supported by the National Natural Science Foundation of China (Grant Nos. 92351301 and 32325002), Dr. Cheng Lei’s team from the Chengdu Biogas Research Institute, Ministry of Agriculture and Rural Affairs, in collaboration with international researchers from the Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Hokkaido University, National Institute of Advanced Industrial Science and Technology (AIST), and Peking University, has achieved a significant breakthrough in understanding microbial interactions driving methane formation. The research, titled "Methanol transfer supports metabolic syntrophy between bacteria and archaea", was published online in Nature on January 30, 2025 (http://www.nature.com/articles/s41586-024-08491-w).
Anaerobic degradation of organic matter to methane is critical for the carbon biogeochemical cycle and is also a major source of atmospheric methane emissions, closely linked to global climate change and renewable energy development. This process typically requires metabolic collaboration between bacteria and methanogenic archaea. Previous studies have identified three interaction modes: interspecies hydrogen transfer, interspecies formate transfer, and interspecies direct electron transfer, collectively termed as "syntrophy". However, only hydrogenotrophic and acetoclastic methanogens were known to participate in these processes, leaving the role of methylotrophic methanogens unclear.
The research team proposed a novel potential metabolic reaction for biological methanol production and validated it through thermodynamic analysis, synthetic co-culture and isotopic tracer, and other techniques. Using the newly isolated bacterial species Zhaonella formicivorans and archaeal species Methermicoccus shengliensis, they demonstrated that the synthetic co-culture achieved formate oxidation to methane via interspecies methanol transfer, establishing a fourth mode of bacterial-archaeal interplay. Genomic, transcriptomic, and metabolomic analyses further revealed a previously unknown glycine-serine cycle responsible for methanol generation.
This discovery advances our understanding of microbial interactions in methane production, expands knowledge of carbon cycling in subsurface environments, and provides a scientific foundation for developing biogas technologies and carbon-neutral strategies. Nature highlighted this work in a research briefing titled "Underground bacteria serve alcohol to methane-making microbes" (http://doi.org/10.1038/d41586-025-00199-9).

Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@donnasnhdiary.org
京ICP备05002826号 文保网安备1101080035号 Copyright 2017 NSFC, All Right Reserved